BP-C1, Meabco’s lead product, is an anti-neoplastic agent with palliative properties and is based on Meabco’s patented polyphenolic technology platform.
BP-C1 is positioned for the global USD 107 billion market of cancer treatment and palliation drugs. The first step is to approach the breast and pancreatic segment of this market, which accounts for 14% of all cancer types and valued at USD 12.3 billion in 2013.
BP-C1 offers a combination of immunomodulation and tumor control in one product. Usually, immunomodulation and tumor control are treated separately and by using two or more drugs.
BP-C1 has been tested in a wide range of animal trials as well as in human clinical trials together demonstrating a high safety profile together with very low toxicity. BP-C1 acts strongly apoptotic, anti-proliferative and anti-angiogenic on tumor cells, while at the same time contributing to anti-cancer immune regulatory activities.
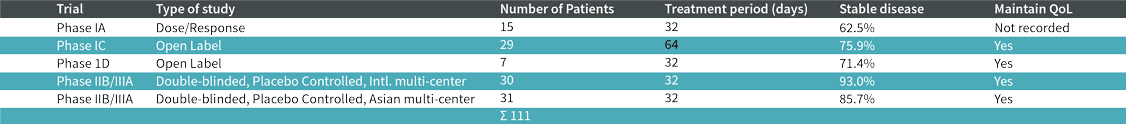
Clinical research
BP-C1 has been tested in close to 300 breast and pancreatic cancer patients in seven countries with promising results. It is noteworthy, that more than 80% of breast cancer patients and more than 60% of pancreatic cancer patients responded to the therapy. BP-C1 is aimed at fighting the cancer combined with strengthening the body’s immune system. Our research has shown that:
- BP-C1 had very low toxicity.
- BP-C1 significantly reducing tumor growth.
- BP-C1 significantly increased the quality of life.
- BP-C1 treatment can be given as an intramuscular injection permitting treatment in a local clinic or in the patient’s own home.